M. William Rochlin
|
Associate Professor
Developmental Neurobiology
Ph.D. 1989, University of North Carolina
Phone: 773.508.2450
Fax: 773.508.3646
E-mail: wrochli@luc.edu
|
RESEARCH INTERESTS
Axons follow predictable and highly circumscribed paths from the time they begin their journey during development to the time they synapse upon their targets. However, if neurons are dissected from an animal and allowed to extend axons in a cell culture setting, the axons grow out nearly randomly. This indicates that the environment of the embryo provides cues that guide the growing tip of the axon, or "growth cone." These cues may be either attractive or repulsive, diffusible or immobile, and they may also influence the branching and bundling of axons. I am interested in understanding the roles of guidance cues and the subcellular mechanisms by which they affect changes in growth cone advance rate and direction. To identify and characterize these cues I co-culture neurons and pathway- or target-containing tissues that appear likely to influence axon extension during embryonic development. I use the sensory (rather than motor) innervation of the rodent tongue as a model system. In most other parts of the body, the sensory axons follow the motor axons until they get near their targets, i.e., the motor axons are the pioneers and the decision-makers. In the tongue, however, the sensory neurons are the pioneers. It is much easier to obtain a purified population of sensory neurons than motor neurons, so the tongue is advantageous for studies of how pioneer axons navigate to their targets.
My initial studies of how axons are influenced by explants of target tissue led to the surprising finding that the pre-tongue secretes a repellent factor (Semaphorin 3A) for sensory axons that will eventually innervate the tongue. The repellent has a role in regulating the medial and anterior progression of lingual sensory innervation during development. If tongue explants are dissected at later stages, they exhibit a decreased repellent activity despite the fact that mRNA for the repellent molecule continues to be elevated and the sensory axons remain sensitive to the repellent in vitro, although the duration of the sensitivity depends on the identity/function of the axons and the neurotrophic factor that is used to stimulate outgrowth.
Currently, we are investigating the roles of neurotrophic factors and Eph/ephrin signaling in axon guidance and target innervation at embryonic and adult stages. Our data show that certain neurotrophins attract neurites and that the balance between the attraction (guided growth) and growth promotion depends on the stage of development. We are determining the distribution of Eph/ephrin signaling molecules and examining the influence of mutations in these signaling systems on pathfinding in vivo.
Ultimately, I hope that the knowledge gained from the study of these developmental processes will contribute to therapies for improving recovery from nervous system injury or disease. Given that embryonic neuronal populations may be used to replace injured adult neurons, understanding how embryonic neurons respond to guidance cues may have clinical applications.
REPRESENTATIVE PUBLICATIONS
Runge, E.M., Hoshino, N., Biehl, M.J., Ton, S., Rochlin, M.W. 2012, NT4 is More Potent than BDNF in Promoting, Attracting, and Suppressing Geniculate Ganglion Neurite Outgrowth. Dev. Neurosci. 34:389-401.
Hoshino, N., Vatterott, P., Egwiekhor, A., and Rochlin, M.W. 2010, BDNF Attracts Geniculate Neurites During Embryonic Targeting. Dev. Neurosci. 32:184–196.
Yamout, A., Spec, A., Kashyap, M., and Rochlin, M.W. 2005, Neurotrophic Factor Receptor Expression and In Vitro Nerve Growth of Geniculate Ganglion Neurons That Supply Divergent Nerves. Dev. Neurosci. 27:288-298.
Vilbig, R., Cosmano, J., Giger, R., Rochlin, M.W. 2004 The Response of Geniculate Neurites to Lingual Chemorepellents Depends on Developmental Stage and Neurotrophic Factors. J. Neurocytol. 33:591-606.
Farbman, A.I., Brann, J.H., Rozenblat, A., Rochlin, M.W., Weiler, E., Bhattacharyya, M. 2004 Developmental expression of neurotrophin receptor genes in rat geniculate ganglion neurons. J. Neurocytol. 33:331-343.
Dillon, T.E., Saldanha, J.F., Kashyap, M., Giger, R., Verhaagen, J., and Rochlin, M.W. 2004, Sema3A Regulates the Timing of Target Contact by Cranial Sensory Axons. J. Comp. Neurol. 470:13-24.
Rochlin, M.W., O'Connor, R., Giger, R.J. Verhaagen, J., Farbman, A.I. 2000, Neuropilin-1 and Sema3A regulate early embryonic geniculate ganglion axon growth. J. Comp. Neurol. 422:579-593.
Rochlin, M.W., Dailey, M.E., and Bridgman, P.C. 1999, Polymerizing Microtubules Activate Site-directed F-Actin Assembly in Nerve Growth Cones. Molec. Biol. Cell 10:2309-2327.
Rochlin, M.W. and Farbman, A.I. 1998, Trigeminal ganglion axons are repelled by their presumptive targets. J. Neurosci. 18:6840-6852.
Rochlin, M.W. and H.B. Peng 1990, The Influence of AChR Clustering Stimuli on the Formation and Maintenance of AChR Clusters Induced by Polycation-coated Beads in Xenopus Muscle Cells. Dev. Biol. 140:27-40.
 |
| BDNF secreted by slow release beads (white) is sufficient to attract neurites from geniculate ganglion explants prepared at stages corresponding to target penetration in vivo. |
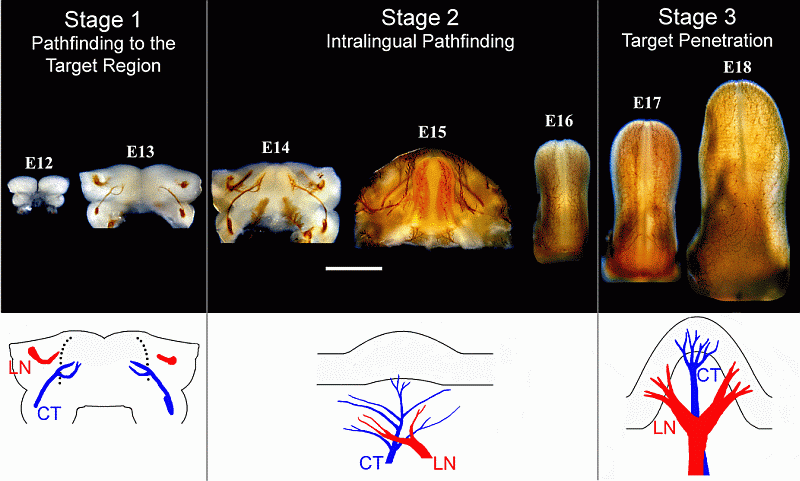
Fig. Nerve staining of rat jaws (embryonic days 12-15) and tongues (embryonic day 16-18) shows the development of the trigeminal lingual nerve (LN) and geniculate chorda tympani (CT) nerve. Ultimately, the trigeminal LN will relay information from the periphery related to touch, pain, and temperature and the geniculate CT will relay taste information. At Stages 2 and 3, the position of the nerve endings in relation to taste papillae is schematically represented (adapted from the work of Albert I. Farbman and Pascal Mbiene).
We identified three stages of innervation distinguished by different responses of geniculate and trigeminal neurites to neurotrophic factors and tongue explants: Stage 1, pathfinding from the ganglion to the pre-tongue; Stage 2, intralingual pathfinding prior to target penetration; and Stage 3, target penetration. Misexpression of Brain-derived Neurotrophic Factor (BDNF) and Neurotrophic Factor 4 (NT4) in vivo (please see the excellent work of Robin Krimm and Christopher Nosrat) affects the guidance of lingual taste axons during Stage 2 and possibly Stage 3, consistent with roles for BDNF and NT4 in guiding afferents during intralingual pathfinding. We are using a tissue culture approach to determine if these neurotrophic factors act directly on neurites or indirectly, e.g., by conferring upon the axons' responsiveness to other factors that act directly as guidance cues or stimulating non-neuronal cells to produce attractants. In addition, we are exploring the influence of neurotrophic factors on the direction, amount, and length of neurite outgrowth at Stage 1 and after birth, throughout the life of the animal. This is especially important to determine in the taste system because taste cells undergo continuous turnover and axon endings must routinely establish new connections or associations.
|
Associate Professor
Developmental Neurobiology
Ph.D. 1989, University of North Carolina
Phone: 773.508.2450
Fax: 773.508.3646
E-mail: wrochli@luc.edu
|
RESEARCH INTERESTS
Axons follow predictable and highly circumscribed paths from the time they begin their journey during development to the time they synapse upon their targets. However, if neurons are dissected from an animal and allowed to extend axons in a cell culture setting, the axons grow out nearly randomly. This indicates that the environment of the embryo provides cues that guide the growing tip of the axon, or "growth cone." These cues may be either attractive or repulsive, diffusible or immobile, and they may also influence the branching and bundling of axons. I am interested in understanding the roles of guidance cues and the subcellular mechanisms by which they affect changes in growth cone advance rate and direction. To identify and characterize these cues I co-culture neurons and pathway- or target-containing tissues that appear likely to influence axon extension during embryonic development. I use the sensory (rather than motor) innervation of the rodent tongue as a model system. In most other parts of the body, the sensory axons follow the motor axons until they get near their targets, i.e., the motor axons are the pioneers and the decision-makers. In the tongue, however, the sensory neurons are the pioneers. It is much easier to obtain a purified population of sensory neurons than motor neurons, so the tongue is advantageous for studies of how pioneer axons navigate to their targets.
My initial studies of how axons are influenced by explants of target tissue led to the surprising finding that the pre-tongue secretes a repellent factor (Semaphorin 3A) for sensory axons that will eventually innervate the tongue. The repellent has a role in regulating the medial and anterior progression of lingual sensory innervation during development. If tongue explants are dissected at later stages, they exhibit a decreased repellent activity despite the fact that mRNA for the repellent molecule continues to be elevated and the sensory axons remain sensitive to the repellent in vitro, although the duration of the sensitivity depends on the identity/function of the axons and the neurotrophic factor that is used to stimulate outgrowth.
Currently, we are investigating the roles of neurotrophic factors and Eph/ephrin signaling in axon guidance and target innervation at embryonic and adult stages. Our data show that certain neurotrophins attract neurites and that the balance between the attraction (guided growth) and growth promotion depends on the stage of development. We are determining the distribution of Eph/ephrin signaling molecules and examining the influence of mutations in these signaling systems on pathfinding in vivo.
Ultimately, I hope that the knowledge gained from the study of these developmental processes will contribute to therapies for improving recovery from nervous system injury or disease. Given that embryonic neuronal populations may be used to replace injured adult neurons, understanding how embryonic neurons respond to guidance cues may have clinical applications.
REPRESENTATIVE PUBLICATIONS
Runge, E.M., Hoshino, N., Biehl, M.J., Ton, S., Rochlin, M.W. 2012, NT4 is More Potent than BDNF in Promoting, Attracting, and Suppressing Geniculate Ganglion Neurite Outgrowth. Dev. Neurosci. 34:389-401.
Hoshino, N., Vatterott, P., Egwiekhor, A., and Rochlin, M.W. 2010, BDNF Attracts Geniculate Neurites During Embryonic Targeting. Dev. Neurosci. 32:184–196.
Yamout, A., Spec, A., Kashyap, M., and Rochlin, M.W. 2005, Neurotrophic Factor Receptor Expression and In Vitro Nerve Growth of Geniculate Ganglion Neurons That Supply Divergent Nerves. Dev. Neurosci. 27:288-298.
Vilbig, R., Cosmano, J., Giger, R., Rochlin, M.W. 2004 The Response of Geniculate Neurites to Lingual Chemorepellents Depends on Developmental Stage and Neurotrophic Factors. J. Neurocytol. 33:591-606.
Farbman, A.I., Brann, J.H., Rozenblat, A., Rochlin, M.W., Weiler, E., Bhattacharyya, M. 2004 Developmental expression of neurotrophin receptor genes in rat geniculate ganglion neurons. J. Neurocytol. 33:331-343.
Dillon, T.E., Saldanha, J.F., Kashyap, M., Giger, R., Verhaagen, J., and Rochlin, M.W. 2004, Sema3A Regulates the Timing of Target Contact by Cranial Sensory Axons. J. Comp. Neurol. 470:13-24.
Rochlin, M.W., O'Connor, R., Giger, R.J. Verhaagen, J., Farbman, A.I. 2000, Neuropilin-1 and Sema3A regulate early embryonic geniculate ganglion axon growth. J. Comp. Neurol. 422:579-593.
Rochlin, M.W., Dailey, M.E., and Bridgman, P.C. 1999, Polymerizing Microtubules Activate Site-directed F-Actin Assembly in Nerve Growth Cones. Molec. Biol. Cell 10:2309-2327.
Rochlin, M.W. and Farbman, A.I. 1998, Trigeminal ganglion axons are repelled by their presumptive targets. J. Neurosci. 18:6840-6852.
Rochlin, M.W. and H.B. Peng 1990, The Influence of AChR Clustering Stimuli on the Formation and Maintenance of AChR Clusters Induced by Polycation-coated Beads in Xenopus Muscle Cells. Dev. Biol. 140:27-40.
 |
| BDNF secreted by slow release beads (white) is sufficient to attract neurites from geniculate ganglion explants prepared at stages corresponding to target penetration in vivo. |

Fig. Nerve staining of rat jaws (embryonic days 12-15) and tongues (embryonic day 16-18) shows the development of the trigeminal lingual nerve (LN) and geniculate chorda tympani (CT) nerve. Ultimately, the trigeminal LN will relay information from the periphery related to touch, pain, and temperature and the geniculate CT will relay taste information. At Stages 2 and 3, the position of the nerve endings in relation to taste papillae is schematically represented (adapted from the work of Albert I. Farbman and Pascal Mbiene).
We identified three stages of innervation distinguished by different responses of geniculate and trigeminal neurites to neurotrophic factors and tongue explants: Stage 1, pathfinding from the ganglion to the pre-tongue; Stage 2, intralingual pathfinding prior to target penetration; and Stage 3, target penetration. Misexpression of Brain-derived Neurotrophic Factor (BDNF) and Neurotrophic Factor 4 (NT4) in vivo (please see the excellent work of Robin Krimm and Christopher Nosrat) affects the guidance of lingual taste axons during Stage 2 and possibly Stage 3, consistent with roles for BDNF and NT4 in guiding afferents during intralingual pathfinding. We are using a tissue culture approach to determine if these neurotrophic factors act directly on neurites or indirectly, e.g., by conferring upon the axons' responsiveness to other factors that act directly as guidance cues or stimulating non-neuronal cells to produce attractants. In addition, we are exploring the influence of neurotrophic factors on the direction, amount, and length of neurite outgrowth at Stage 1 and after birth, throughout the life of the animal. This is especially important to determine in the taste system because taste cells undergo continuous turnover and axon endings must routinely establish new connections or associations.

